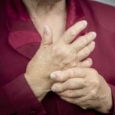
Glucosamine

|
|
Quick navigation |
What are Glucosamine's other names?
No other names for this supplement.What is Glucosamine's recommended dosage?
- Recommended daily intake: 900 - 1500 mg
- Recommended daily doses: 3
What supplements interact with Glucosamine?

|